Professor Daniel Ken Inaoka

Speciality / Research theme / Keywords
Tumour biology, Molecular biology, Structural biology, Medicinal ⅽhemistry, ParasitologySupervision
Master’s programmeDoctoral ProgrammeQualifications
PhD, Pharmaceutical Sciences
Research gate account links
https://www.researchgate.net/profile/Daniel_Inaoka
Affiliation(s)
Department of Molecular Infection Dynamics, Institute of Tropical Medicine, Nagasaki University Visiting Researcher, Department of Biomedical Chemistry, School of International Health, Graduate School of Medicine, The University of Tokyo
Background
I was born and lived 21 years in Brazil, an endemic country for Chagas disease and Leishmaniasis. I studied at the Faculty of Pharmacy from Universidade Federal do Rio Grande do Norte. In 2000, I came to Japan and under the support of Mombukagakusho Scholarship I obtained Master and PhD degree in Pharmaceutical Sciences at Graduate School of Pharmaceutical Sciences, The University of Tokyo. During my Master and Doctoral course I have analyzed the biochemical and structural biological properties of dihydroorotate dehydrogenase, a potential drug target from Trypanosoma cruzi involved in pyrimidine de novo biosynthesis. In 2005, I moved to Graduate School of Medicine, The University of Tokyo supported by Postdoctoral Fellowships for Foreign Researchers (JSPS) to start research in structural biology and drug design targeting the unique energy metabolism found in Trypanosomatids and Helminthes. Since 2007, I become Assistant Professor of Department of Biomedical Chemistry from Graduate School of Medicine at The University of Tokyo to research molecular and biochemical parasitology and drug design targeting enzymes involved in microaerophilic mitochondrial metabolism from protozoan parasites and helminthes. I have expertise in the field of biochemistry, parasite metabolism, structural biology and drug design.
Teaching
I worked as part-time lecturer for practical class in biophysics at Teikyo University, between 2002 and 2005. I was also in charge of practical class in biochemistry at School of International Health, The University of Tokyo, from 2007 to 2016. At TMGH, Nagasaki University, I will be teaching part of Basic Human Biology coordinated by Prof. Kita and Prof. Kamiya.
Research
Based in keywords such as “Parasite”, “Mitochondria”, “Host Environment Adaptation”, “Ubiquinone”, “Energy Metabolism”, “Biochemistry”, “Drug Target” and “Drug Development”, my current research area include Trypanosomatids and Apicomplexan parasites, Helminthes and Tumor Microenvironment.
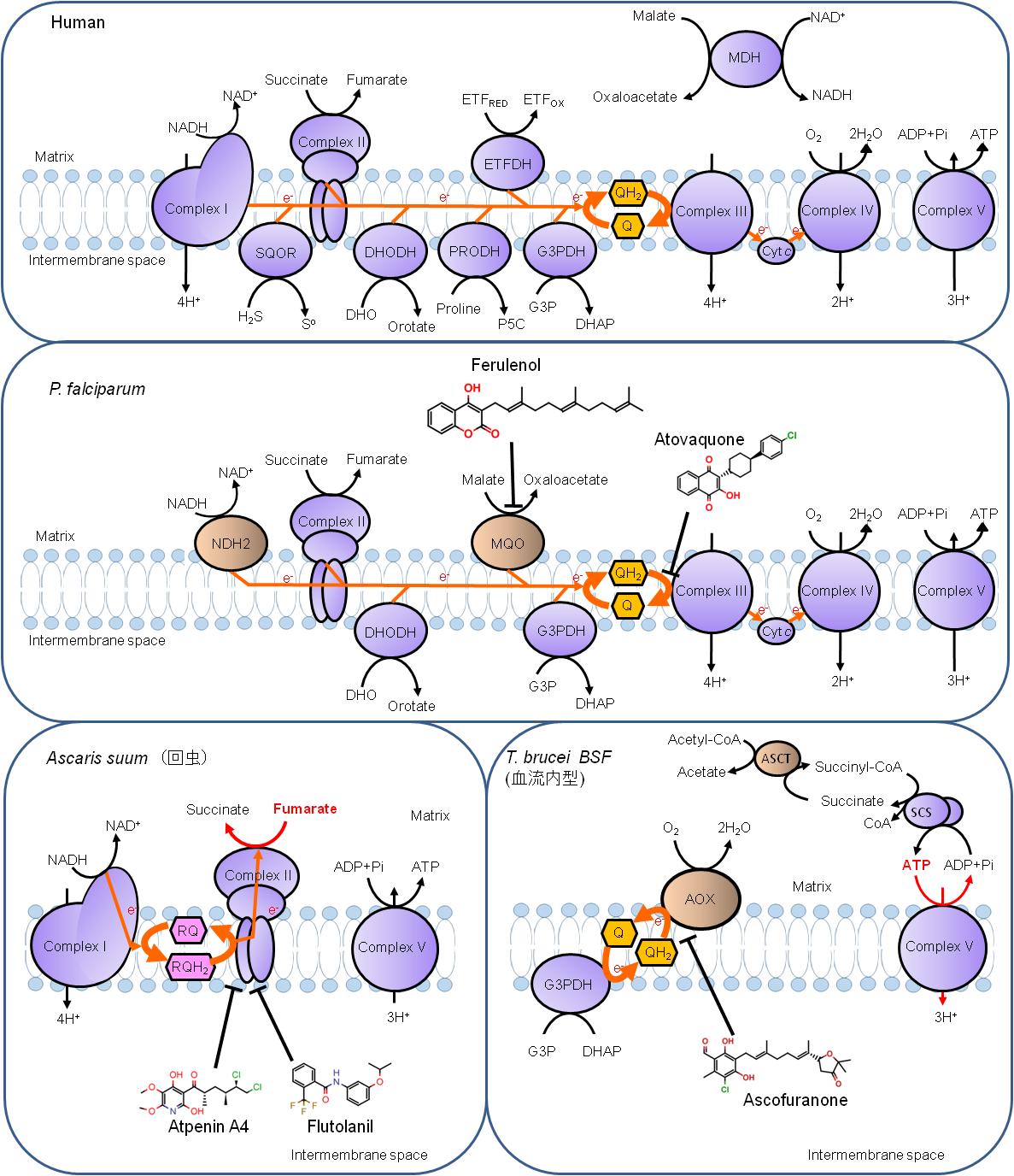
Differences in the mitochondrial energy metabolism between human (top), P. falciparum (middle), Ascaris suum (left bottom) and T. brucei blood stream forms (BSF). Complex I: NADH dehydrogenase; Complex II: Succinate dehydrogenase; Complex III: Quinol-cytochrome c (Cytc) reductase; Complex IV: Cytochrome c oxidase; Complex V: ATP synthase; SQOR: Sulfide:quinone oxidoreductase; DHODH: Dihydroorotate (DHO) dehydrogenase; PRODH: Proline dehydrogenase; G3PDH: Glycerol-3-phosphate (G3P) dehydrogenase; ETFDH: Electron transfer flavoprotein (ETF) dehydrogenase; MDH: Malate dehydrogenase; Q: Ubiquinone; QH2: Ubiquinol; NDH2: Type-II NADH dehydrogenase; MQO: Malate:quinone oxidoreductase; RQ: Rhodoquinone; RQH2: Rhodoquinol; AOX: Alternative oxidase; ASCT: Acetate:succinate CoA transferase; SCS: Succinyl-CoA synthethase; CoA: Coenzyme A. Enzymes not conserved in human genome is highlighted in light orange color.
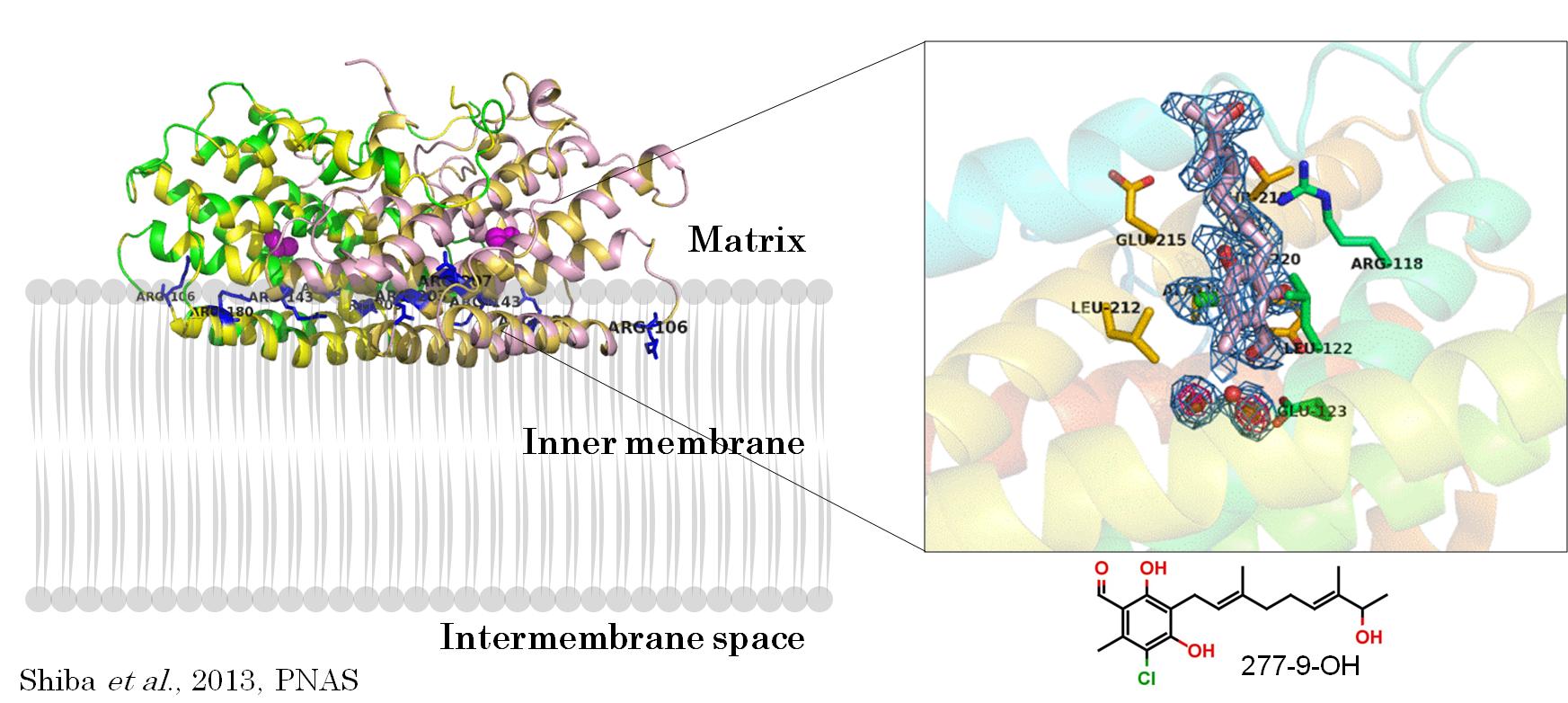
Crystal structure of T. brucei Alternative Oxidase (AOX) in complex with 277-9-OH, an ascofuranone derivative. AOX is a key enzyme in energy metabolism of plants, fungi and T. brucei. It is essential in BSF and required to maintain the redox balance within glycosomes, a Trypanosomatid-specific organelle.
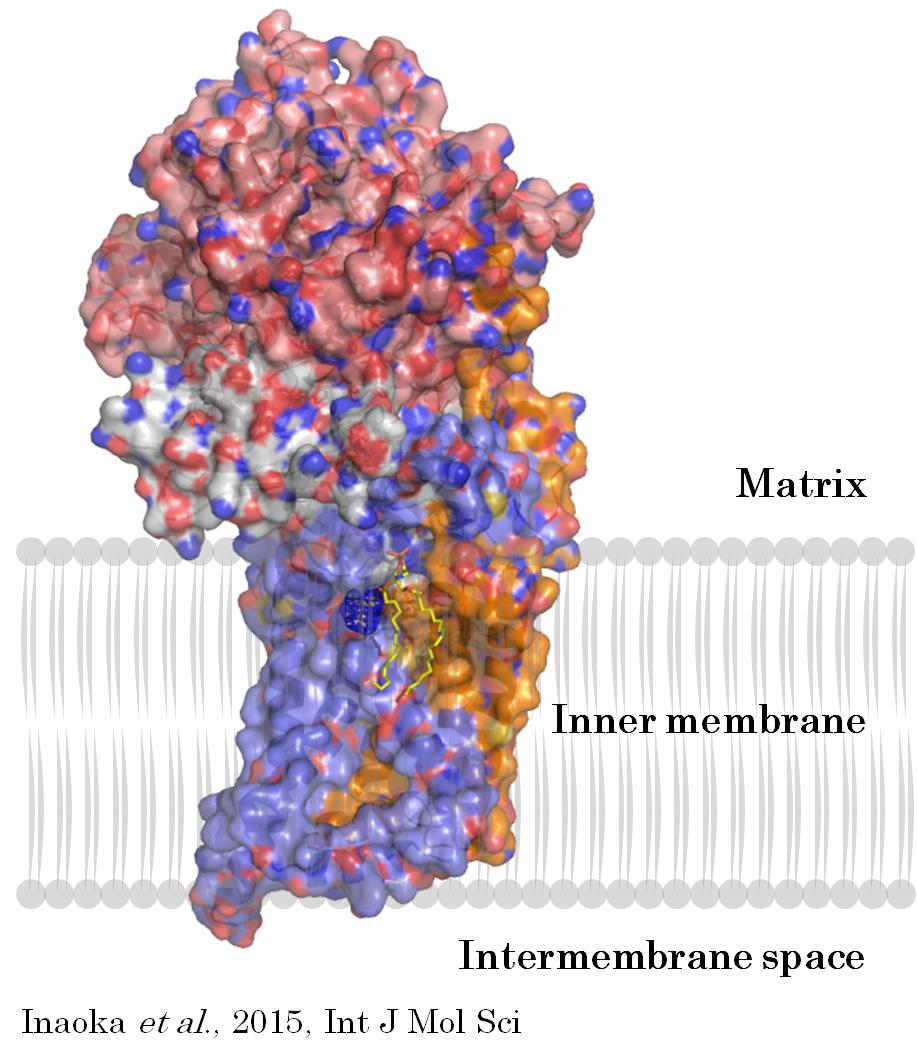
Crystal structure of mitochondrial Quinol:fumarate reductase (QFR or Complex II) from A. suum. This enzyme is a heterotetramer composed by flavoprotein (salmon color), iron-sulfur protein (white), large (purple) and small (orange) cytochrome b subunits. In the small intestine where oxygen concentration is extremely low, this enzyme catalyzes the last step in fumarate respiration and essential for adaptation to host environment. Inhibitors of QFR are active against the helminthes that lives in microaerophilic environment such as adult stage of A. suum, Haemonchus contortus, and Dirofilaria immitis.
The country/countries where you work currently
- Japan
- Indonesia
- El Salvador
Five Key Recent Publications
- Enkai S, Kouguchi H, Inaoka DK, Shiba T, Hidaka M, Matsuyama H, Sakura T, Yagi K, Kita K. Killing Two Birds with One Stone: Discovery of Dual Inhibitors of Oxygen and Fumarate Respiration in Zoonotic Parasite, Echinococcus multilocularis. Antimicrob Agents Chemother. 2023; 67(3):e0142822.
- Kabongo A T, Acharjee R, Sakura T, Bundutidi GM, Hartuti E D, Davies C, Gundogdu O, Kita K, Shiba T, Inaoka DK. Biochemical characterization and identification of ferulenol and embelin as potent inhibitors of malate:quinone oxidoreductase from Campylobacter jejuni. Front Mol Biosci. 2023; 10:1095026.
- Komatsuya K, Sakura T, Shiomi K, Omura S, Hikosaka K, Nozaki T, Kita K, Inaoka DK. Siccanin Is a Dual-Target Inhibitor of Plasmodium falciparum Mitochondrial Complex II and Complex III. Pharmaceuticals (Basel). 2022; 15(7):903.
- Hidayati A R, Melinda, Ilmi H, Sakura T, Sakaguchi M, Ohmori J, Hartuti E D, Tumewu L, Inaoka DK, Tanjung M, Yoshida E, Tokumasu F, Kita K, Mori M, Dobashi K, Nozaki T, Syafruddin D, Hafid A F, Waluyo D, Widyawaruyanti A. Effect of geranylated dihydrochalcone from Artocarpus altilis leaves extract on Plasmodium falciparum ultrastructural changes and mitochondrial malate: Quinone oxidoreductase.Int J Parasitol Drugs Drug Resist. 2023; 21:40-50.
- Talaam KK, Inaoka DK, Hatta T, Tsubokawa D, Tsuji N, Wada M, Saimoto H, Kita K, Hamano S. Mitochondria as a Potential Target for the Development of Prophylactic and Therapeutic Drugs against Schistosoma mansoni Infection. Antimicrob Agents Chemother. 2021; 65(10):e0041821.
